Your experts for skin transplantation
Hier erläutern wir Ihnen die verschiedenen Möglichkeiten der Hautverpflanzung. Lernen Sie die Vor- und Nachteile der SkinDot-Transplantation, Spalthaut-Transplantation, Kulturhautverfahren im Labor gezüchteter Zellen und die Möglichkeiten des Ersatzes tieferHautschichten (dermale Ersatzverfahren) kennen. Welche Hauttransplantation eignet sich wofür?
Ask our experts
Dermal replacement procedures
For deep wounds with complete loss of all skin layers, a collagen-based dermal substitute can be grafted into the wound. This collagen matrix acts as a skin shifting layer. A thin layer of skin must then be transplanted over the dermal substitute.
Culture skin method
Since the 1980s, skin cells can be grown in the laboratory. The skin cells grown (cultured) in the laboratory can be inserted into the wound as cell layers or sprayed onto the wound.
Split skin graft
For extensive burns and very superficial wounds, so-called split-thickness skin grafting is the treatment of choice. Split skin consists of an ultra-thin skin graft of the thickness of only 0.2 mm.
SkinDot skin graft
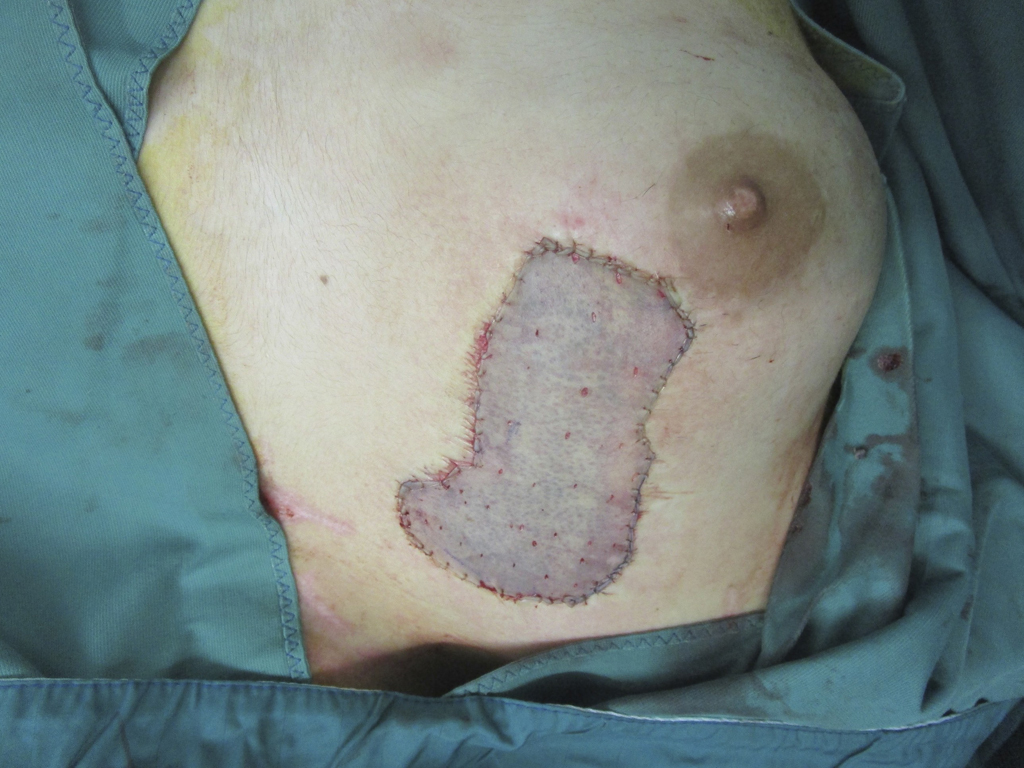
In the case of a deep wound with a loss of the entire skin, skin replacement with so-called full-thickness skin provides the best result. Full-thickness skin grafts are very stable due to their dermal shifting layer and contain all skin appendages.
Split skin graft
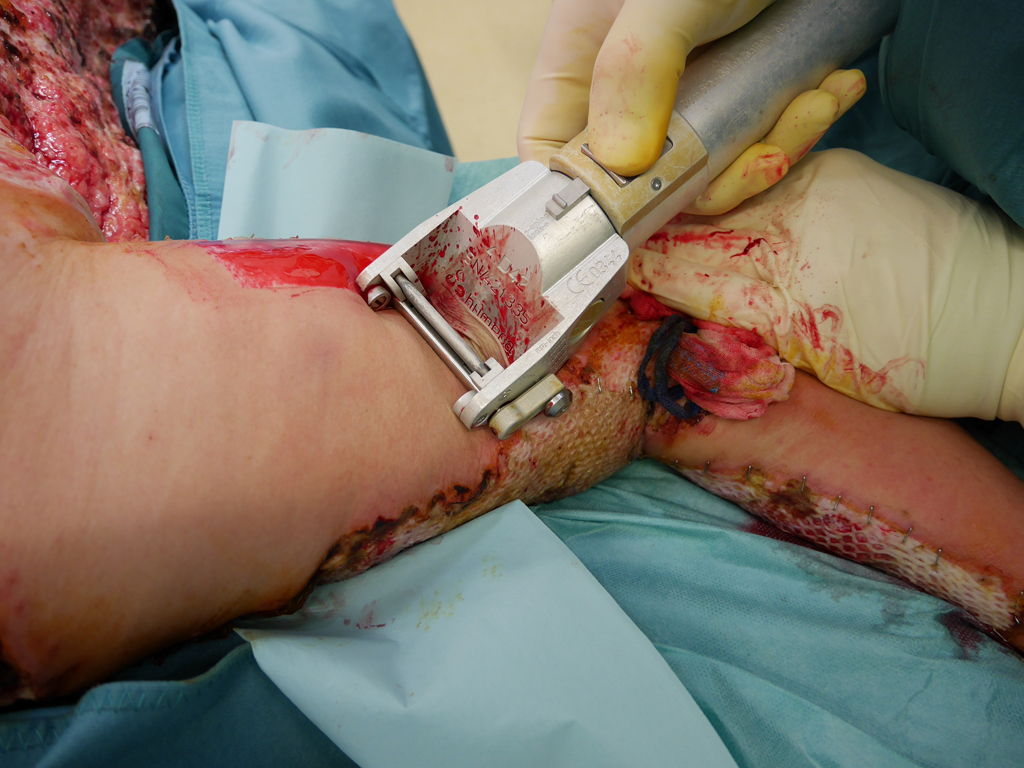
In principle, split skin, full skin (SkinDot) or cultured skin can be transplanted as part of skin transplantation. In the case of full thickness skin, the entire epidermis and dermis are transplanted, whereas in the case of split thickness skin, only the epidermis and the upper parts of the dermis are transplanted. A 0.2 mm thick layer of skin (split skin) is removed with a compressed air or electrodermatome and transplanted onto the wound. In some emerging and developing countries, a hand dermatome or the scalpel is still used to remove the split skin. Since no skin appendages are transplanted due to the thickness of the graft, wound defects treated with split-thickness skin do not show hair growth. Furthermore, they must be continuously lubricated, since the sebaceous gland function is also missing. The sensation of pain is severely limited, and temperature regulation via perspiration is not possible.
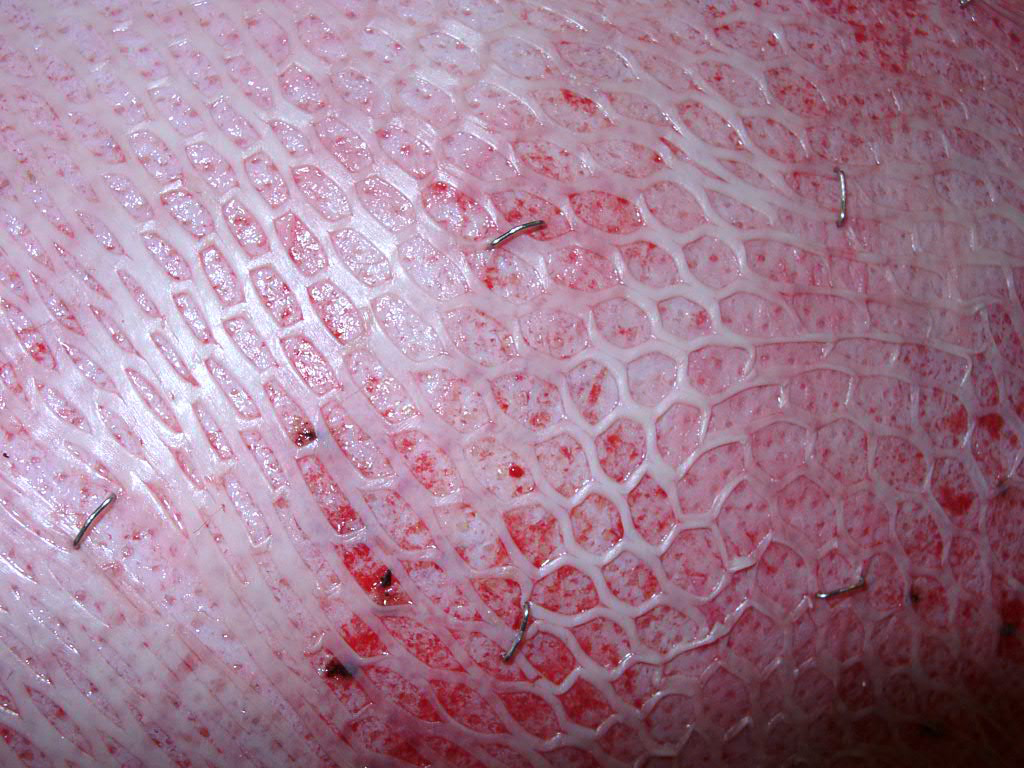
For surface enlargement, a mesh pattern can be cut into the split skin and expanded (mesh). After “meshing,” the split skin takes on the appearance of a lattice- or net-like graft. If the split skin is transplanted un-meshed, it is referred to as a sheet graft. Sheet grafts vary in thickness from approximately 0.125 to 0.75 mm (0.005 to 0.030 inch). Depending on the amount of dermis contained, they are classified as thin (0.125 to 0.3 mm = 0.005 to 0.012 inch), medium (0.3 to 0.45 mm = 0.012 to 0.018 inch), or thick (0.45 – 0.75 =0.018 to 0.030 inch) split-skin grafts.
.
Mesh graft
Split skin grafting using mesh graft technique is the most commonly used skin grafting technique. Here, the skin graft can be expanded at a ratio of 1:1, 1:1.5, 1:3, and 1:6. The more limited the donor area, the larger the expansion must be to cover all wound areas. One surgical option to circumvent the problem of the limited donor area is therefore to expand the split skin to a greater extent. In the so-called Alexander technique, the widely expanded split skin is combined with foreign skin. Thus, expansion rates of the meshed split skin up to 1:9 are possible, although rarely performed.
An alternative technique to expand the split skin is the Meek method (see below). The Meekgraft technique is a safe method to use when the split skin expansion rates are large. It is established in many burn centers worldwide because of its simpler grafting and lower fragility compared with large-mesh mesh grafts and is often preferred to the widely expanded mesh graft technique. Raff et al. also demonstrated that when the Meek technique is used up to a burned body surface area of 75%, the remaining unburned skin is sufficient as a donor area and only needs to be harvested once to avoid additional application of cultured skin.
The larger the meshes and the corresponding grid or mesh spacing or expansion, the longer the re-epithelialization of the grafted wound. Reepithelialization occurs starting from the edges of the grafted split skin, with keratinocytes confluent in the skin gaps. The gaps in the meshed graft allow drainage of wound secretions, preventing the risk of seroma or hematoma formation under the skin graft. Consistent immobilization of the grafted areas to avoid shear forces is a prerequisite for safe healing of the skin grafts. Shear forces prevent the capillaries from sprouting into the transplanted skin, which can survive only about 3-5 days without its own blood supply via diffusion processes from the wound. Prevention of shear forces can be achieved by using an overknot dressing or by applying negative pressure therapy over the graft.
.
The disadvantage of the mesh graft is that a cosmetically and functionally unfavorable result is often achieved because the mesh texture remains permanently visible. Too much removed split skin can be wrapped in a sterile compress moistened with NaCL at 4°C in the refrigerator, stored in a sterile container, and still transplanted 7-10 days later. Therefore, a possible donor site that is sometimes forgotten is the skin of amputated extremities, which can be used for defect coverage with a time delay.
| Meshgraft | Vorteil | Disadvantage |
|---|---|---|
| Skin quality of the graft | Poor, as no skin appendages in the graft, no stem cells present, no dermale Verschiebeschicht | |
| Healing of the graft | Since thin graft fast healing time | |
| Scarring | Grid or network structure visibler | |
| Donor area | Large-scale decrease at the thighs and on the back possible, but texture and pigment changes on the donor area | |
| OP-Zeit | First removal of the split skin and then transplantation, if necessary in parallel by two surgical teams |
Sheet graft
If sufficient donor sites are available, the harvested split skin can be used for grafting without expansion. The size ratio in this case is 1:1, and the graft is called a sheetgraft. Sheetgraft grafts are cosmetically favorable because these grafts present a smooth skin relief postoperatively and typical texture patterns that occur after all size expansion methods (meshgraft, meekgraft) are absent. Due to the often limited donor area, they are often used for smaller cosmetically exposed areas, and in the severely burned patient they are reserved for skin grafting of the face, genitals, and hands. It is important to note the inherent shrinkage tendency of all split-thickness skin grafts, even unmeshed ones, depending on the thickness of the graft, which can significantly reduce the often excellent initial cosmetic result. In facial transplants, graft placement should be based on the aesthetic zones of the face. The risk of fluid retention beneath the sheet graft with impending graft loss can be countered by scarification. This involves stitching the seetgraft with a scalpel, as fluid accumulation under the graft prevents the capillaries from sprouting.
Alternatively, the sheet graft can be meshed at a 1:1 ratio but not expanded or minimally expanded. However, after 1:1 meshening, the sheet graft becomes a mesh graft under strict classification. Sheet grafts have the lower shrinkage tendency compared to mesh grafts and produce the aesthetically better results. While most authors consider meshgraft grafts to be contraindicated in the face, there are publications on cosmetically flawless transplantation of minimally expanded meshgraft grafts in the forehead.
.
Fixation of the sheet graft is by either staples, sutures, or fibrin glue, and there are combinations of all three fixation techniques. The fixation technique has no effect on the postoperative outcome. In contrast, an overknot or negative pressure dressing has an effect on the graft success. In an overknot dressing, opposing sutures of approximately 10-15 cm in length are applied to the wound edges. A foam pad, cut to fit the size of the wound, is applied to the thin layer of fat gauze overlying the graft and then secured to the pad under compression by tying the ends of the sutures.
.
| Sheetgraft | Vorteil | Disadvantage |
|---|---|---|
| Skin quality of the graft | Poor, as no skin appendages in the graft, no stem cells present, no dermal displacement layer | |
| Healing of the graft | Since thin graft fast healing time | |
| Scarring | Initially good, later often hypertrophic scarring especially at the margins, no Skin tags present | |
| Donor area | Large-scale decrease at the thighs and on the back possible, but texture and pigment changes on the donor area | |
| OP-Zeit | First removal of the split skin and then transplantation, if necessary in parallel by two surgical teams |
Meek technique
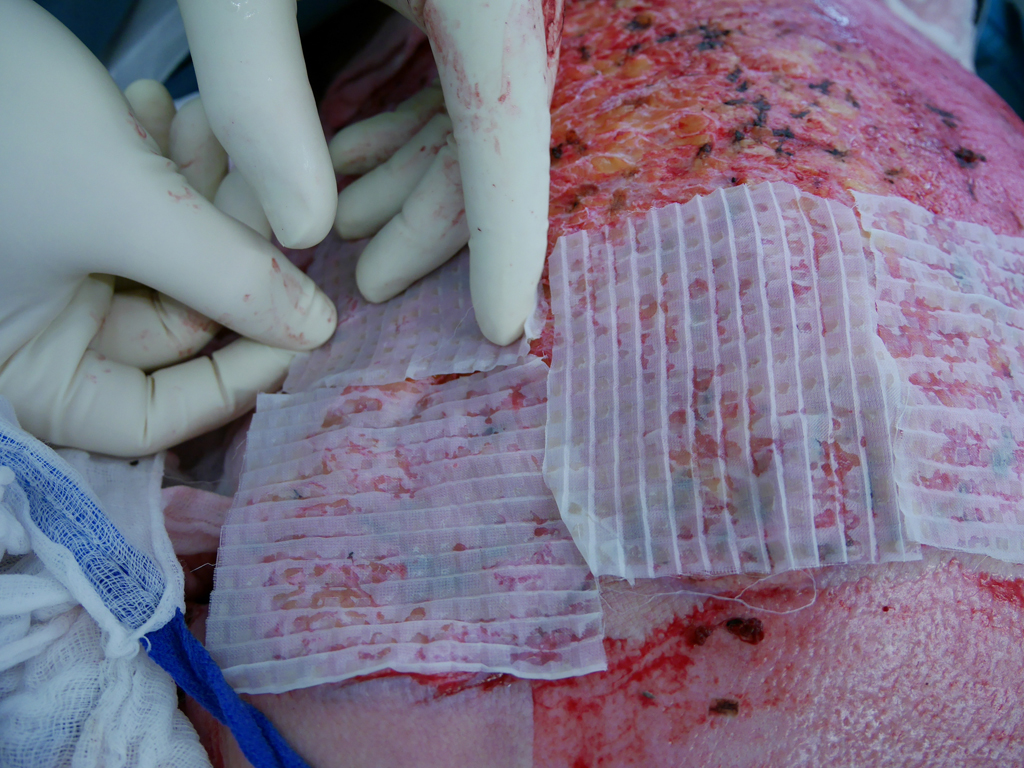
One surgical option to circumvent the problem of limited donor area is greater expansion of split skin. In the so-called Alexander technique, the expanded split skin is combined with foreign skin. Thus, expansion rates of the meshed split skin up to 1:9 are possible, although rarely performed. An alternative technique to expand the split skin is the method according to Meek. This method, which is safe to use for large expansion rates of the split skin, is established in many burn centers worldwide because of its simpler grafting and lower fragility compared with the large mesh grafts, and is preferred to the mesh graft technique for very large burns. Raff et al. also demonstrated that up to a burned body surface area of 75%, the remaining unburned skin is sufficient as a donor area and only needs to be used once to avoid additional application of cultured skin. Cicero Parker Meek (1914 – 1979) worked as a general surgeon at Aiken County Hospital in South Carolina, USA, whose special interest was the treatment of severely burned patients. In his article “Successful Microdermagrafting using the Meek-Wall Microdermatome” published in the American Journal of Surgery in 1958, he described for the first time a device-based method for producing “postage stamp-like” small skin grafts. His basic reasoning was that the keratinocytes of grafted skin islands confluence from the graft margin to the periphery. From this, he concluded that the longer the margins of the grafts, the faster the wound healing of transplanted areas would have to be.
By means of a mathematical approximation, he presented the principle that the smaller the transplanted squares, the larger the sum of the edges of the square grafts. From this reasoning, he constructed the Meek-Wall device for micrograft transplantation first presented in the aforementioned article. The device first presented in the original 1958 article already had the 13 cutting blades driven by an electric motor. It also describes the cork plate as a carrier for the graft and how the device works. Initially, Meek soaked the small (1/16 inch = 4 mm) skin islands in plasma and then applied them by hand to parachute silk, which he grafted directly to the wound bed. In February 1963, he and S.P. Wall, the engineer with whom he had co-designed the device, registered the Meek- Wall Dermatome with the United States Patent Office as a “microdermatome.” In 1965, Meek published a second article in which he reported on his experiences with the new technique and described the individual steps of the Meek transplantation in detail for the first time. However, Meek’s method fell into oblivion internationally after the introduction of the meshgraft technique in 1964, as the strapless, lattice-shaped mesh grafts could be produced more quickly and much more easily and thus initially gained acceptance. Chinese experiences of transplanting split skin in an island shape were not published internationally. It was not until the early 1990s that the Meek technique was revived and technically improved by Dutch surgeons at the Red Cross Hospital in Beverwijk. A spray adhesive specially developed for the adhesion of the split skin to the cork flaps and new nylon pleats were introduced. The clinical results of this modified Meek technique were first published by Kreis et al. in 1993. In the article, the authors recommended for the first time a combination of Meek and allograft (foreign skin) at high expansion rates.
One week after Meek grafting, the nylon pleats are carefully peeled off and foreign skin is grafted over the adherent nonconfluent islets. In another 1994 article on the Meek technique by Kreis et al, the authors compared the expansion rates of mesh and Meek grafts in an in vitro experiment and concluded that an expansion of 1:6 using the mesh technique was equivalent to an expansion of 1:4 using the Meek technique. A further modification was published by Raff et al. in 1996. The authors stated that for expansion rates up to 1:6, additional transplantation of foreign skin is not necessary. In 1997, at the suggestion of surgeon Robert Kreis, pleats with an expansion rate of 1:3 were produced for the first time. The last modification was made in 2001 by Dr. Abdul Reda Lari from the Al Babtain Burn Center in Kuwait City. He had the idea of replacing the pneumatic motor (which drives the 13 blades) with a hand crank, as compressed air is not available in many countries, making the device more affordable for emerging and developing countries. The Meek technique allows definitive wound management by split-thickness skin grafting up to a burned body surface area of 75% without additional grafting of cultured skin [3,9]. Surgical treatment may be necessary in several steps due to intraoperative blood loss in extensive burns. Depending on the patient’s constitution and intensive medical preparation (up-transfusion), coverage of up to 25% VKOF with simultaneous necrosectomy is possible in one surgical session. Higher coverage rates can be achieved if the patient has already undergone necrosectomy, the wounds have been appropriately debrided and conditioned by temporary wound care. If there are no complications, the islets adhere firmly to the wound bed no later than seven days after transplantation. Complete re-epithelialization of the gaps between the grafted islets is achieved within two weeks at expansion rates up to 1:3. Kreiss et al. reported a re-epithelialization time of five weeks at an expansion of 1:9 in combination with foreign skin.
| Meekgraft | Advantage | Disadvantage |
|---|---|---|
| Skin quality of the graft | Poor because no skin appendages in graft, no stem cells present, no dermal shift layer | |
| Healing of the graft | Since thin graft fast healing time | |
| Scarring | Island structure visible | |
| Donor area | Small area removal possible on all remaining healthy skin areas | |
| OP time | Complicated technique, long manufacturing time of the grafts necessary |
Your attending physician is not yet familiar with the new, innovative SkinDot procedure? Please inform him/her about the possibility of treating your skin wound with the new SkinDot procedure. We will be happy to advise you and perform the SkinDot procedure on you (if indicated). We look forward to hearing from you!
Skin Transplantation beyond
Skin Transplantation 2.0