Your Skin Transplant Experts
Hier erläutern wir Ihnen die verschiedenen Möglichkeiten der Hautverpflanzung. Lernen Sie die Vor- und Nachteile der SkinDot-Transplantation, Spalthaut-Transplantation, Kulturhautverfahren im Labor gezüchteter Zellen und die Möglichkeiten des Ersatzes tieferHautschichten (dermale Ersatzverfahren) kennen. Welche Hauttransplantation eignet sich wofür?
Ask our experts
Dermal replacement procedures
For deep wounds with complete loss of all skin layers, a collagen-based dermal substitute can be grafted into the wound. This collagen matrix acts as a skin shifting layer. A thin layer of skin must then be transplanted over the dermal substitute.
Culture skin method
Since the 1980s, skin cells can be grown in the laboratory. The skin cells grown (cultured) in the laboratory can be inserted into the wound as cell layers or sprayed on the wound.
Split skin graft
For extensive burns and very superficial wounds, so-called split-thickness skin grafting is the treatment of choice. Split skin consists of an ultra-thin skin graft of the thickness of only 0.2 mm.
SkinDot skin graft
In the case of a deep wound with a loss of the entire skin, skin replacement with so-called full-thickness skin provides the best result. Full-thickness skin grafts are very stable due to their dermal shifting layer and contain all skin appendages.

Warning
They may contain disturbing photos.
Dermal Replacement Procedures
.
The skin consists of a thin outer skin layer (epidermis) and a thicker skin layer (hypodermis, dermis). The dermis represents the shifting layer of the skin and provides the elasticity of the skin.
In full-thickness wounds and deep burns (IIb°-III°), the dermis is destroyed.
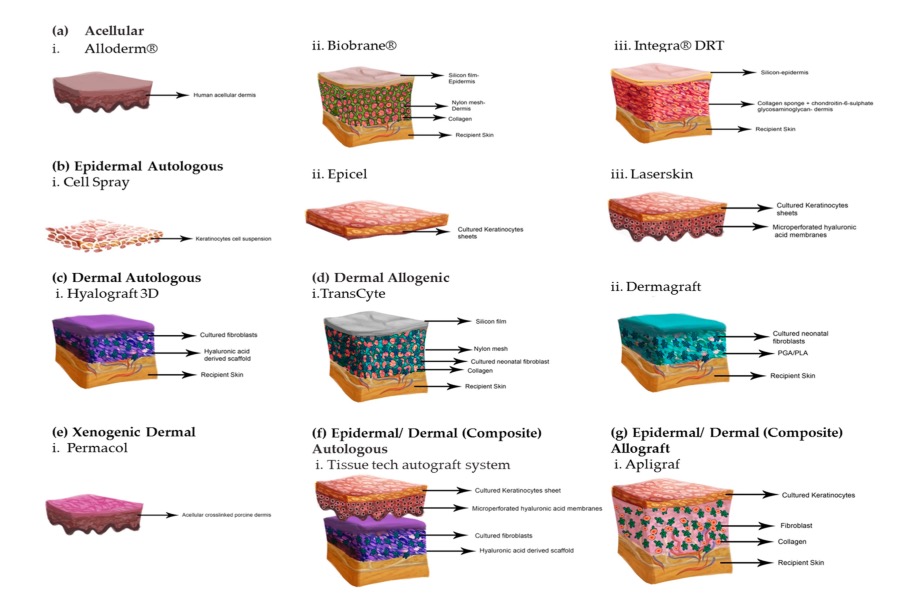
In SkinDot full-thickness skin, the dermis is already included. Another, older procedure for dermis replacement is the transplantation of a dermal replacement layer (matrix) into the wound. The matrix consists of collagen, glycoosaminoglycan, elastin, and/or hyaluron. These dermal substitute layers are commercially available medical devices that require special approval.
Dermal replacement procedures differ in their one- or two-stage surgical application. In single-stage application, the dermal substitute matrix is transplanted into the wound and a thin split-thickness skin graft is placed over the matrix in the same surgical procedure. Smallest blood vessels (capillaries) must sprout into the skin graft so that the split skin is supplied with blood and nourished. When transplanting a dermal substitute layer, these blood vessels must first grow through the matrix before they reach the graft. Therefore, the matrix must not be too thick. The advantage of the single-stage procedure is therefore relativized by the fact that only very thin replacement layers can be used. However, the quality of the dermal shift layer is characterized precisely by a certain minimum thickness. The second surgical principle of the dermal replacement procedure is therefore the two-stage surgical procedure. First, a thicker replacement layer is transplanted and the patient waits until sufficient capillaries have sprouted. After approximately 10-14 days, the split skin is then transplanted in a second procedure.
Engineered dermal Matrices
Integra Artificial Skin® was the first artificially generated matrix commercially available as a dermal replacement layer. Integra Artificial Skin® consists of a three-dimensional pore structure on a glycosaminoglycan collagen type I chondroitin base and a silicone membrane as a barrier against infection and dehydration of the matrix.
After the capillaries have sprouted, special skin cells (fibroblasts) migrate in with the blood, leading to the remodeling of the replacement matrix into a near-equivalent endogenous dermis.
When blood circulation is safe after 10-14 days, the silicone membrane is removed and replaced by split skin. Much faster blood flow to the dermal replacement layer can be achieved by the additional application of negative pressure. Negative pressure application also has the advantage of minimizing shear forces, preventing secretion accumulation under the graft, and sealing the matrix against external influences. Other commercially available dermis replacement materials are now available that differ in terms of their three-dimensional pore structure and composition. In addition, further new matrices are under scientific investigation and some of them are already in phase II of the necessary registration studies with application on patients in multicenter observational studies, such as Novomaix® or Denovoskin®. Purely synthetic materials as dermal substitutes are increasingly used in clinical practice. These purely synthetic materials impress with their easy storage, non-cooled transport and lower costs compared to the expensive biological matrices made of bovine collagens and/or bovine elastin.
.
| Product name | Egineered Dermal Matrices | |
|---|---|---|
| Biobrane® | Smith & Nephew AG | Three-dimensional nylon mesh made of silicone and collagen |
| Alloderm® | Lifecell Corporation | Acellular allogeneic dermis |
| Xenoderm® | MBP GmbH | Acellular xenogeneic matrix |
| Integra® | Integra Life Science Corporation | Three-dimensional glycosaminoglycan matrix of collagen type I and chondroitin-6-sulfate plus silicone layer |
| Matriderm® | Dr. Suwelak Skin & Healthcare AG | Three-dimensional matrix of bovine collagen types I, III and V and elastin |
| Laserskin® | Fidia Biopolymers | Perforated three-dimensional matrix of hyaluronic acid |
Egineered dermal-epidermal Matrices
These constructs attempt to grow the all skin layers in the laboratory in order to be able to transplant them as a full-layer skin substitute. The attempt to combine laboratory-grown skin cells (primarily keratinocytes and fibroblasts) with various support materials has been pursued by numerous international research groups. Yannas and Burke first introduced a skin equivalent in 1989 by centrifuging keratinocytes and fibroblasts and seeding them in a collagen-glycosaminoglycene matrix, which initially showed promise in animal studies but failed when applied to patients.
Comparable research results led to the commercially available product Apligraf® at the end of the 1990s. This involves laboratory-grown cratinocytes from umbilical cord blood and foreskin on a bovine collagen matrix.
The desire for artificial matrices as full-thickness skin substitutes remains high in medicine, especially for the therapy of chronic wounds. A relatively new product against this background is Orcel®, which also contains a combination of neonatal keratinocytes and fibroblasts, but is grown in the laboratory on a gel-like basis without a pore structure. Which dermo-epidermal replacement skin will ultimately prevail on the market cannot be estimated at present. New medical trends are moving in the direction of “custom made” matrices, in which the matrix is grown on a three-dimensional matrix using the patient’s own skin cells cultivated in the laboratory after a skin biopsy, but a disadvantage that cannot currently be overcome is the still long cell cultivation period of up to four weeks. During this period, the cultured skin cells are highly susceptible to infection, and the patient must have the lost skin barrier replaced with an artificial replacement skin made of plastic until the matrix from the laboratory is fully grown.
It is currently impossible to predict how long it will take before a full-length replacement from the laboratory can be used in patients. Also, important long-term studies regarding graft resilience, scarring, and skin quality of this lab-grown replacement skin are still lacking.
| Product name | Egineered Dermal Matrices | |
|---|---|---|
| Apligraf® | Trademark of Novartis AG | Human allogeneic neonatal keratinocytes in a three-dimensional collagen type I gel derived from allogeneic neonatal fibroblasts. |
| Orcel® | Ortec International Incorporation | Human allogeneic cultured keratinocytes and allogeneic fibrobalsts in a collagen gel scaffold. |
| Transcyte® | Smith&Nephew AG | Neonatal allogeneic human fibroblasts.n |
Your attending physician is not yet familiar with the new, innovative SkinDot procedure? Please inform him/her about the possibility of treating your skin wound with the new SkinDot procedure. We will be happy to advise you and perform the SkinDot procedure on you (if the indication is suitable). We look forward to your appointment!
Skin Transplantation beyond
Skin Transplantation 2.0