Your Skin Transplantation Experts
Here we explain the different options for skin grafting. Get to know the advantages and disadvantages of full-thickness skin transplantation, split-thickness skin transplantation, cultured skin procedures, spray cell technique and dermal substitute matrix in detail. Which transplant procedure is suitable for which indication?
Ask our experts
Dermal replacement procedures
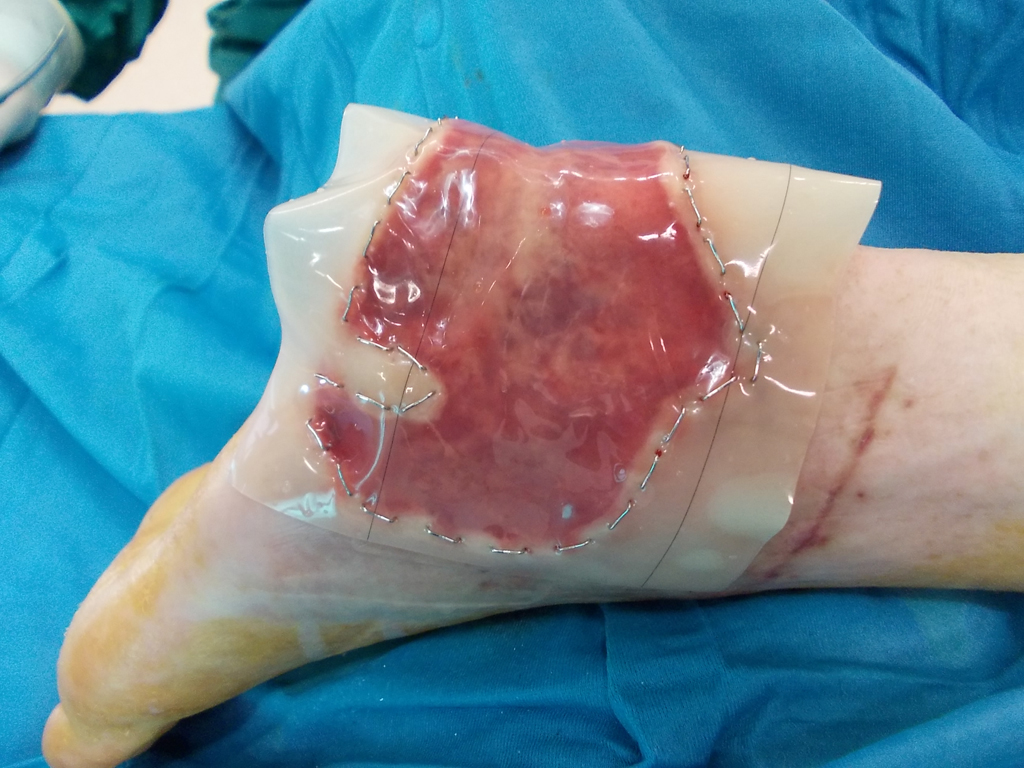
For deep wounds with complete loss of all skin layers, a collagen-based dermal substitute can be grafted into the wound. This collagen matrix acts as a skin shifting layer. A thin layer of skin must then be transplanted over the dermal substitute.
Culture skin method
Since the 1980s, skin cells can be grown in the laboratory. The skin cells grown (cultured) in the laboratory can be inserted into the wound as cell layers or sprayed on the wound.
Split skin graft
For extensive burns and very superficial wounds, so-called split-thickness skin grafting is the treatment of choice. Split skin consists of an ultra-thin skin graft of the thickness of only 0.2 mm.
SkinDot skin graft
In the case of a deep wound with a loss of the entire skin, skin replacement with so-called full-thickness skin provides the best result. Full-thickness skin grafts are very stable due to their dermal shifting layer and contain all skin appendages.
Dermal Replacement Procedures
.
The dermis represents the shifting layer of the skin and provides the elasticity of the skin. Deep wound defects and IIb°-III° thermal lesions are associated with a loss of the dermis.
In SkinDot full-thickness skin, the dermis is already included.
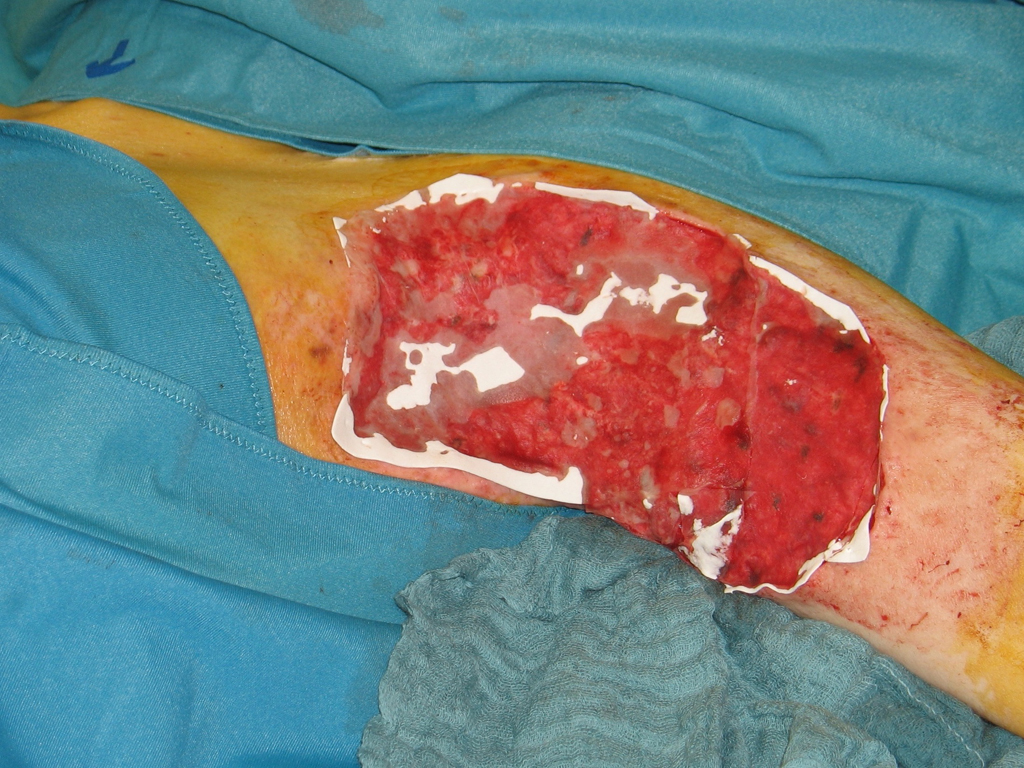
Another, older procedure for dermis replacement is transplantation of a dermal replacement matrix into the wound. Dermal replacement matrices consist of collagen, glycosamines, elastin, or hyaluron.
Dermal replacement procedures differ in their one- or two-stage surgical application. In single-stage application, the dermal replacement matrix is transplanted into the wound and a thin split-thickness skin graft is grafted over the matrix in the same surgical procedure. However, in single-stage grafting of a dermal replacement layer, the capillaries must first sprout through the matrix before reaching the graft. Therefore, the matrix must not be too thick. The advantage of the single-stage procedure is therefore relativized by the fact that only very thin matrices can be used. However, the quality of the dermal shift layer is characterized precisely by a certain minimum thickness. The second surgical principle of the dermal replacement procedure is therefore the two-stage procedure. In this procedure, a thicker replacement matrix is transplanted first. If vascularization is sufficient, the split skin is transplanted in a second procedure after approximately 10-14 days.
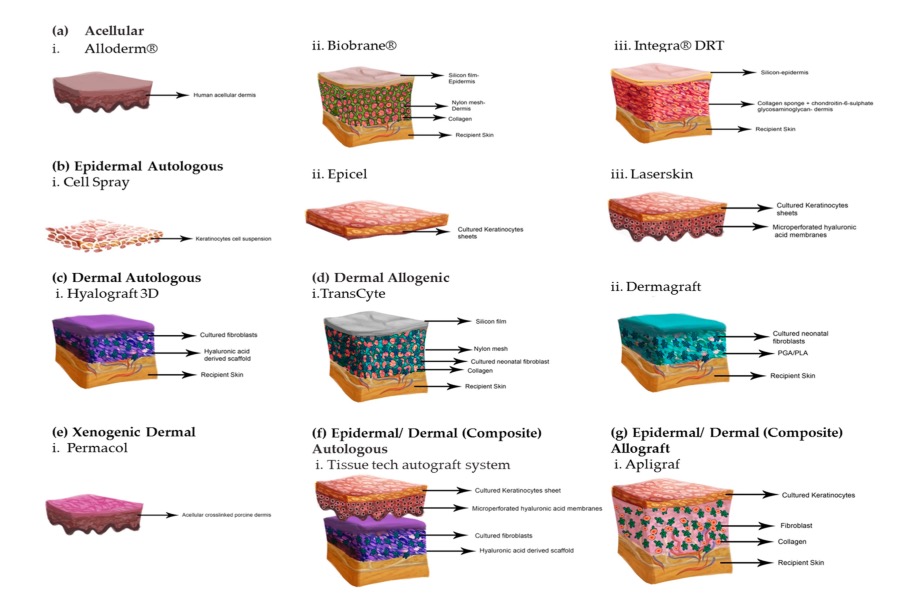
Engineered dermal Matrices
Integra Artificial Skin® was the first laboratory-generated bilaminar matrix used as a dermis substitute. It is based on a three-dimensional glycosaminoglycan-based pore structure and a silicone membrane as an epidermal barrier against infection and dehydration of the matrix. With the sprouting of capillaries, fibroblasts migrate, leading to the remodeling of the replacement matrix into an approximately equivalent endogenous dermis. After sufficient vascularization, split skin or laboratory-cultured cells are transplanted onto the Integra Artificial Skin® Matrix after removal of the silicone membrane.
.
Much faster vascularization can be achieved by the additional application of a negative pressure (e.g. VAC®) to the Integra Matrix. Negative pressure has the advantage of minimizing shear forces, preventing potential seroma or hematoma, and sealing the matrix.
The sealed wound chamber reduces the penetration of germs into the matrix and thus the risk of infection. The initial hope that additional skin appendages would sprout into the matrix has not been realized.
Die Transplantation einer dünnen dermalen Ersatzmatrix plus Spalthaut kann
einzeitig als „Single step“ Operation durchgeführt werden, allerdings mit Abstrichen bezüglich
der Matrixdicke und damit Einbußen in der Qualität der dermalen Verschiebeschicht. Andere
kommerziell erhältliche Dermis Ersatzmaterialien schlossen an sich der Integra Artificial Skin®
Matrix an, die sich vor allem bezüglich ihrer Porenstruktur und Zusammensetzung
unterscheiden.
Im Moment befinden sich weitere neue Matrices bereits in der Phase II der
notwendigen Zulassungsstudien mit Anwendung am Patienten im Rahmen von Multicenter
Anwendungsbeobachtungen, wie z.B. Novomaix® oder Denovoskin®. Rein synthetische Materialien als dermaler Ersatz finden im klinischen Alltag immer mehr Einsatz. Diese rein
synthetischen Materialien bestechen durch Ihre einfache Lagerung, ungekühlten Transport und
niedrigere Kosten gegenüber den biologischen Matrices aus bovinen Kollagenen und/oder
Elastin.
| Product name | Engineered Dermal Matrices | |
|---|---|---|
| Biobrane® | Smith & Nephew AG | Three-dimensional nylon mesh made of silicone and collagen |
| Alloderm® | Lifecell Corporation | Acellular allogeneic dermis |
| Xenoderm® | MBP GmbH | Acellular xenogeneic matrix |
| Integra® | Integra Life Science Corporation | Three-dimensional glycosaminoglycan matrix of collagen type I and chondroitin-6-sulfate plus silicone layer |
| Matriderm® | Dr. Suwelak Skin & Healthcare AG | Three-dimensional matrix of bovine collagen types I, III and V and elastin |
| Laserskin® | Fidia Biopolymers | Perforated three-dimensional matrix of hyaluronic acid |
Engineered dermal-epidermal Matrices
The attempt to combine cultured autologous keratinocytes with various allogeneic materials has been pursued by numerous research groups worldwide. Yannas and Burke first presented a skin equivalent in 1989 by centrifugation of trypsinized keratinocytes and fibroblasts in a collagen-glycosaminoglycene matrix, which showed promise in animal studies but failed to gain clinical acceptance. Comparable results resulted in the late 1990s in the commercially available product Apligraf®, a combination of allogeneic human neonatal keratinocytes and fibroblasts on a bovine collagen matrix. The demand for three-dimensionally engineered engieered dermal-epidermal matrices remains high, especially for the therapy of chronic wounds. A relatively new product against this background is Orcel®, which also contains a combination of neonatal allogeneic keratinocytes and fibroblasts, but is based on a gel-like base without a pore structure. Which dermo-epidermal matrix will ultimately prevail on the market cannot be estimated at present. Decisive factors are not only the best short- and long-term results, but also factors such as product launch, marketing and costs. New trends are moving towards “custom made” matrices, where autologous cultured keratinocytes and fibroblasts are inoculated into the matrix after a skin biopsy. However, a disadvantage that cannot be overcome at present is the still long cultivation time of three to four weeks. Models to accelerate the cultivation time by means of special bioreactors or mechanical transduction are the subject of current intensive medical-scientific research. Regardless of the product and the different matrices and the question whether allogeneic or autologous cells or cell components are advantageous, there is still a lack of information regarding graft resilience, scar formation and skin quality. scar formation and skin quality, numerous application studies are still lacking.
| Product name | Engineered Dermal Matrices | |
|---|---|---|
| Apligraf® | Trademark of Novartis AG | Human allogeneic neonatal keratinocytes in a three-dimensional collagen type I gel derived from allogeneic neonatal fibroblasts. |
| Orcel® | Ortec International Incorporation | Human allogeneic cultured keratinocytes and allogeneic fibrobalsts in a collagen gel scaffold. |
| Transcyte® | Smith&Nephew AG | Neonatal allogeneic human fibroblasts. |
Skin Transplantation beyond
Skin Transplantation 2.0